One of the most frequent decisions when choosing milk is whether to purchase organic or conventional milk. What is the difference between organic and conventional milk? Is the higher price of organic milk worthwhile?
What a cow eats can be passed through to its milk
What a cow is fed can pass through to her milk.(1) Therefore, it is important to consider the feeds, medicines, and possibly even hormones administered to dairy cattle when comparing conventional and organic milk.
The table below summarizes the practices allowed during the management of organic and conventional dairy cattle.
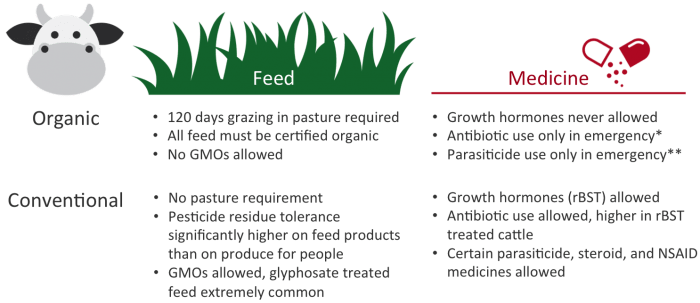
What do conventional dairy cattle eat?
There are two considerations for the hays and grains that make-up conventional dairy cattle feed: residual pesticide levels and whether the components in the feed are from genetically engineered (GMO) crops.
Potentially high pesticide residue on conventional dairy cattle feed
Dairy cattle are exposed to a wide range of other pesticide residues through the feed they consume, and there are no routine practices in place to monitor those pesticide levels in milk.
The EPA sets allowable pesticide levels, or tolerances, for individual crops. The tolerance for a pesticide varies not only by crop, but also vary across different components of a single crop.
Often, the tolerance levels set for hay and forage are higher than the tolerance levels for produce.
Some pesticides ingested by dairy cattle can be passed on to their milk, and though the residual pesticides in milk are not routinely tested by the USDA, the agency periodically includes analyzing the pesticide residues in milk in its Pesticide Data Program (PDP) as a “special project.”(2)
The results from the available years vary widely, as shown by the graph below.
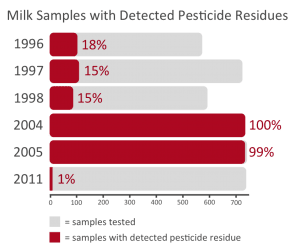
In 2011, only 1% of the tested milk samples had any detectable pesticide residues. However, in 2005, 99% of the milk samples tested had detectable pesticide residues and 85% of the tested milk samples contained trace amounts of DDE (a metabolite of DDT).
It would be a mistake to look at the 2011 test results and conclude that there has been an overall reduction in residual pesticides in milk.
The most reasonable assumption is that there is a wide variance in pesticide residues in the feed dairy cattle consume.
It should be noted that none of these detected pesticide residues were above the set EPA tolerances.
Conventional dairy cattle feed typically comes from genetically engineered crops
The FDA considers genetically engineered crops (GE, also called GMO) to be as safe as any other crop that is grown using traditional breeding practices because GE crops are nutritionally similar to non-GE crops (i.e. similar levels of vitamins, minerals and other nutrients) and do not introduce new allergens into a food.(3)
However, the majority of corn (92% USA / 81% Canada) and soy (94% USA / 62% Canada) grown in North America are GE to be herbicide tolerant (typically glyphosate, trade name Roundup®) and/or insect resistant.(4)
This means that GE crops likely contain herbicide residues and feed crops often have substantially higher allowable tolerance levels for pesticides than the foods we eat.
The EPA tolerance levels for glyphosate vary greatly: non-grass animal feed has an allowable glyphosate tolerance of 400 ppm while pop corn has an allowable glyphosate tolerance of 0.1 ppm.(5)
Even though glyphosate is the most used chemical in agriculture, it is not included on the USDA’s PDP testing program, so no data exists publicly on residual glyphosate levels in milk.(6)
However, 90% of soy beans were found to have residual levels of glyphosate when tested as a “special project” by the USDA in 2011.(7)
Key takeaways: There are no testing procedures in place to routinely monitor pesticide residues in milk. Hays and other animal feeds often have higher allowable pesticide tolerance levels than the foods we eat.
Also, conventionally raised dairy cattle frequently eat genetically engineered feed that likely contains glyphosate and other herbicide residues.
What do organic dairy cattle eat?
Organic dairy cattle must be pastured and housed on land that qualifies for organic certification. They must have access to pasture during the entire grazing season and have at least 120 days grazing on pasture to be certified organic.(8)
Additionally, any feed and hay the cattle are fed must be certified organic, which prohibits the use of genetic engineering.
While this does not mean the feed the organic dairy cattle are eating is pesticide free (organic does not mean pesticide free), it does mean that there are no organophosphate pesticide residues or glyphosate (herbicide, trade name Roundup®) residue on the feed.
Key takeaways: Organically raised dairy cattle eat organically grown feed. Organic feed is not a product of genetic engineering and is free of most pesticide residues, including organophosphate pesticides and glyphosate.
What medicines are given to conventional dairy cattle?
Medicine use in conventional dairy farming is a common practice, with antibiotics and parasiticides as two of the most commonly administered classes of drugs.
When a medicine is approved for dairy cows, a residue tolerance in milk is typically established by the FDA.
Additionally, if applicable, withhold periods are determined where milk must be discarded until residual medicine levels are below the FDA’s tolerance level.(9)
Antibiotic use in conventional dairy farming
Infections, such as mastitis, are common among dairy cattle and antibiotics are often administered. Some antibiotics have specific withhold periods, where the milk from the affected cow is not allowed to be sold. As an example, amoxicillin has a withhold period of 60 hours after last treatment.(10)
The FDA’s Grade “A” Pasteurized Milk Ordinance for requires testing of every truckload of milk for residues of the most commonly used class of antibiotics on diary farms (Beta-lactam).(1)
In the FDA’s Center for Veterinary Medicine’s 2013 Milk Drug Residue Survey, less than 1% of all tested milk samples tested positive for Beta-lactam antibiotics.(11)
However, not all antibiotics have withhold periods. Monensin, an antibiotic that has an FDA-approved claim to “increase milk production efficiency,” has no withhold requirements and is routinely mixed into dairy cattle feed.(12-14) And because monensin is not a Beta-lactam antibiotic, it is not one of the antibiotics that is routinely tested for in milk.(1)
This means that there is a possibility that conventional milk in the US may contain non-Beta-lactam antibiotic residues. More importantly, the overuse and misuse of antibiotics in livestock is contributing to antibiotic resistance. Note: Did you know that 80% of the antibiotics sold in the United States and Canada are for livestock?
Parasiticides and other medicines use in conventional dairy farming
Other medicines used in conventional dairy farming include parasiticides, non-steroidal anti-inflammatory drugs, and antihistamines. These drugs are allowed for routine administration and have allowable tolerance levels in milk.(15)
Since there are no routine testing requirements for medications in milk other than Beta-lactam antibiotics, there is no publicly available data on the residual levels (if any) of these medicines in milk.
Growth hormone use in conventional dairy farming
The use of recombinant bovine seratropin (rBST) in dairy cattle has been banned in the European Union and Canada due to animal welfare concerns.(16)
However, rBST is still used in some US dairy farms and the most recent estimate by the USDA indicates that 17% of all dairy cattle in the US are treated with rBST.(17) While milk produced from animals treated with rBST does not have to be labeled, milk from cows not treated with rBST can be labelled “rBST-free.”(18)
The FDA approved recombinant bovine seratropin (rBST) also called recombinant bovine growth hormone (rBGH), for use in dairy cows in 1993. The genetically engineered growth hormone was marketed as a way to increase a cow’s milk production by 10-15%.(16)
However, there are trade-offs with this increase in milk production. Dairy cattle treated with rBST have an increased incidence of mastitis, causing a corresponding increase in antibiotic use.(16)
While consuming rBST does not affect a person directly because the human body does not recognize cow proteins, milk from rBST treated cows does contain elevated levels of insulin-like growth factor 1 (IGF-1) which is identical to human IGF-1.
Though there are no studies that link consuming milk and dairy products with elevated levels of IGF-1 with adverse health outcomes, there are also no studies demonstrating their safety. And there are studies that have linked high blood levels of IGF-1 with an increased risk of developing prostate cancer in men and breast cancer in women.(18)
Key takeaways: Other than Beta-lactam antibiotics, there are no testing procedures in place to routinely monitor medicine residues in milk. It is concerning to not know whether or not you are exposed to even a low concentration of a medication.
Growth hormones are no longer commonly used in conventional dairy farming in the United States, and are illegal in Canada and Europe. However, because of animal welfare concerns and a paucity of data demonstrating safety of consuming milk with elevated levels of IGF-1, milk from cows treated with rBST should be avoided.
What medicines are given to organic dairy cattle?
The USDA guidelines for organic dairy cattle management require preventative health practices to minimize infections and infestations in the herds. However, they also provide and require treatment options in the event that a dairy cow has a life-threatening illness.
Antibiotic use in organic dairy farming
If an organic dairy cow has a life threatening infection, or is suffering, antibiotics must be administered. However, the milk from the cow may not be sold or fed to organic calves, and the cow must be transferred to a non-organic dairy farm.(8)
Parasiticide use in organic dairy farming
Three synthetic parasiticides are allowed for use in the case of health care emergencies and require a 90-day withholding period for lactating dairy cattle.(4)
Organic dairy farming does not allow the use of synthetic growth hormones
Administration of synthetic hormones to encourage growth or increase milk output is prohibited in organic dairy cattle.(4)
Final thoughts on choosing organic or conventional milk
Conventional and organic dairy cattle are raised differently. There are significant differences in the feed and medicines allowed in each. These differences are important because residual pesticides and medicines can be passed from the cow to their milk.
Avoid conventional milk from cows treated with rBST
Both the unknown health impact of consuming milk with higher levels of IGF-1 and concerns about the animal welfare of the cows treated with rBST warrant avoiding milk and dairy products from cows treated with rBST. Buy milk with the label “no rBST” or “no artificial growth hormone.”
Be aware that conventional milk may contain glyphosate
Conventional milk may contain glyphosate and other herbicide residues commonly used on genetically engineered crops and conventional farming practices.
Choose organic milk to avoid medicine and pesticide residues
If you would like to ensure the milk you and your family consume is free of medicine and organophosphate pesticide residues, it is best to choose organic milk.
Pin article for later:

References:
- Grade “A” Pasteurized Milk Ordinance, FDA 2011 Revision (link)
- USDA PDP Special Projects, Milk, 1996, 1997, 1998, 2004, 2005 and 2011 (link)
- Consumer Info About Food from Genetically Engineered Plants, FDA Website (link)
- Non-GMO Project, GMO acreage in 2014: Corn (link), and Soy (link)
- Electronic Code of Federal Regulations (eCFR), Glyphosate; tolerances for residues, §180.364 (link)
- EPA Pesticides Industry Sales and Usage 2006 and 2007 Market Estimates, 2011. (link)
- USDA PDP Special Projects, Glyphosate, 2011 (link)
- USDA Guidelines for Organic Certification of Dairy Livestock (link)
- Milk Drug Residue Sampling Survey, FDA, March 2015 (link)
- Amoxi-Mast website, Merck Animal Health (link)
- National Milk Drug Residue Data Base Fiscal Year 2017 Annual Report, Center for Veterinary Medicine, FDA, 2013 (link)
- Rumensin website, Elanco (link)
- Rumensin New Animal Drug Application (NADA Number 095-735), FDA, (link)
- FDA Clarifies, Monenesin Safe for Cattle, Goats, Drovers Website (link)
- FDA Memoranda of Information, M-I-05-5: Tolerance and/or Safe Levels of Animal Drug Residues in Milk, 2005 (link)
- Recombinant Bovine Growth Hormone, OLR Research Report, 2007 (link)
- Bovine Somatotropin (bST) – Possible Increased Use of Antibiotics to Treat Mastitis in Cows October 30, 2013, FDA (link)
- What to Eat, Marion Nestle, 2006 (link)
This article was originally published on January 18th, 2017. This updated article has been edited for readability and to update content.

Kelli @ Hungry Hobby
Wednesday 24th of January 2018
Really interesting and super well researched!
Meredith
Wednesday 24th of January 2018
Thanks so much Kelli!
Jennifer
Saturday 6th of January 2018
Meredith, I am new to your site and I appreciate your thoughtful and well cited approach. I wonder what you think of this study (citation to press release below) from Washington State University that did not confirm presence of glyphosate in human milk, even when there were detectable levels in the same subject's urine? My supposition is that if glyphosate does not bioaccumulate in human milk it is likely not to accumulate in cow's milk either, although I certainly can't find any studies on the levels of glyphosate in cow's milk (which in itself is somewhat worrisome considering higher levels of pesticide is allowable in animal feed than human food).
I would love to buy organic milk for a multitude of reasons but must prioritize food decisions to fit the budget. Is there a different source of information I should consider?
Washington State University. "U.S. breast milk is glyphosate free: Study is first independently verified look for the presence of Roundup ingredient in human milk." ScienceDaily. ScienceDaily, 23 July 2015
Meredith
Saturday 6th of January 2018
Hi Jennifer.
Thank you for such a thoughtful question and forwarding the WSU article (which I had not previously read). The study showed no detectable levels of glyphosate were found the human milk and were confirmed by a second, independent, lab... which is important as Monsanto was the other lab analyzing the human milk.
I believe your supposition is accurate... if glyphosate does not bioaccumulate in human milk, it is unlikely to bioaccumulate in cows milk. In fact, a paper was published last month by researchers in Germany that demonstrated that "no glyphosate residues were detected in (dairy cow) milk" (ref: https://www.ncbi.nlm.nih.gov/pubmed/29110579).
If your goal is to avoid glyphosate residue in food, it is likely better to prioritize buying organic or pesticide-free produce that consistently tests positive for pesticides.
Thank you for reading and let me know if you have any other questions! Meredith
Brynn at The Domestic Dietitian
Tuesday 2nd of January 2018
Such a great post, so informative and love the facts and resources.
Meredith
Monday 15th of January 2018
Thank you Brynn!